Pemigatinib: A Targeted Treatment Option for Cholangiocarcinoma and Beyond
The era of personalised medicine is transforming how we treat cancer, and Pemigatinib is at the forefront of this movement. As a targeted therapy, Pemigatinib represents a major leap in managing cancers with FGFR2 genetic alterations—especially cholangiocarcinoma, a rare and aggressive cancer of the bile ducts.
Approved by the U.S. FDA and other global regulatory bodies, Pemigatinib is one of the first medications specifically designed to target fibroblast growth factor receptor (FGFR) mutations in cancer patients. This advancement offers a new lifeline for individuals with limited treatment options and poor prognosis.
In this article, we delve deep into what Pemigatinib is, how it works, who it’s for, its clinical evidence, and the availability of trusted formulations in India like PEMAZYRE 4.5MG / 9MG / 13.5 MG TAB.
Understanding Cholangiocarcinoma: A Rare Cancer with Limited Options
Cholangiocarcinoma, commonly known as bile duct cancer, arises in the slender tubes that carry bile from the liver to the small intestine. It’s a challenging cancer to treat due to:
- Late diagnosis
- Rapid progression
- High resistance to conventional therapies
Most patients are diagnosed at an advanced or metastatic stage, where surgery is no longer viable. Until recently, treatment options were limited to systemic chemotherapy, which had modest benefits and substantial toxicity.
This scenario changed with the introduction of FGFR-targeted therapy—a new class of drugs that address a specific genetic driver of this cancer.
What Is Pemigatinib?
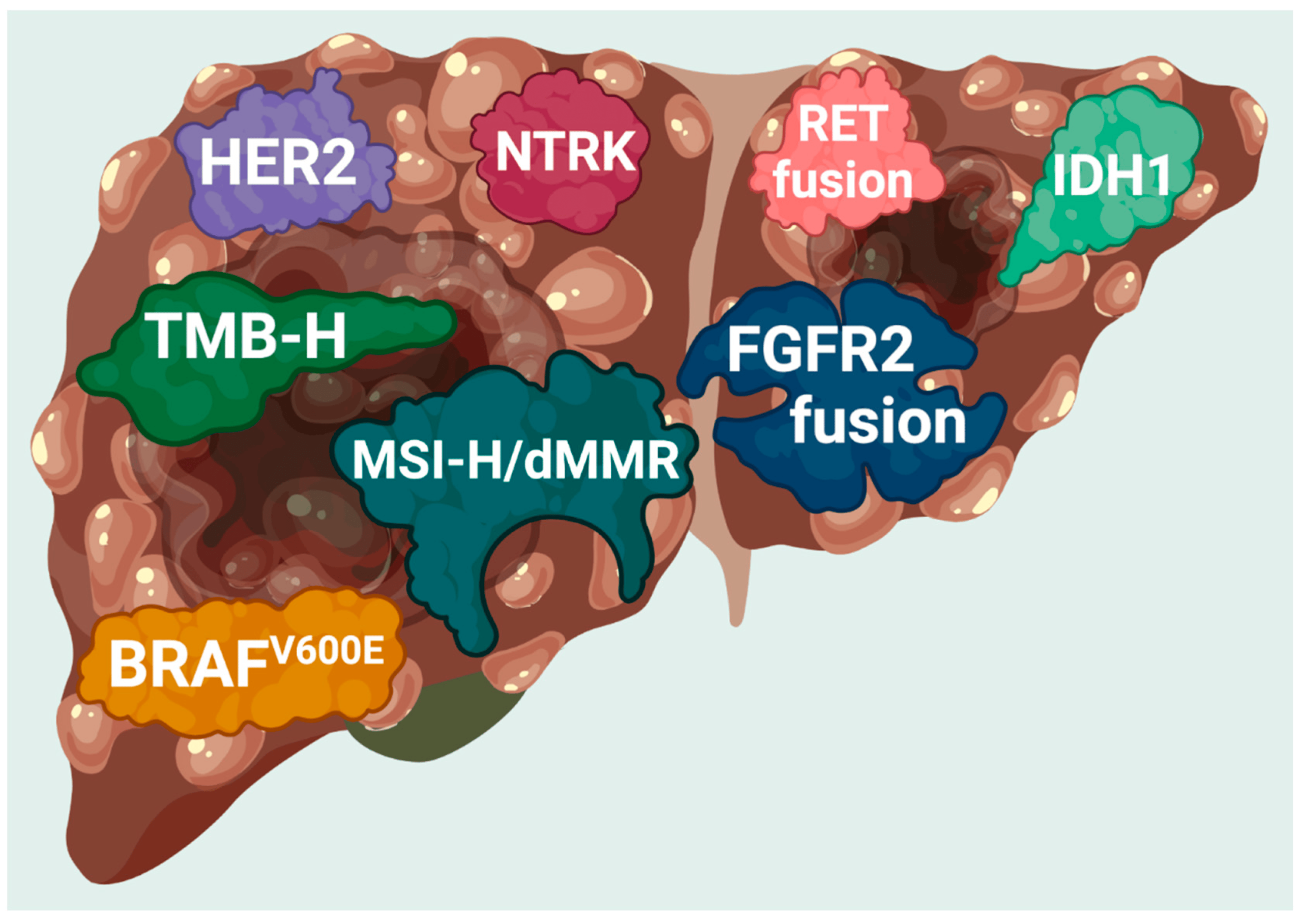
Pemigatinib is an oral FGFR inhibitor that blocks the fibroblast growth factor receptors (FGFR1, FGFR2, and FGFR3). These receptors play a critical role in cell growth, differentiation, and survival. In certain cancers—especially intrahepatic cholangiocarcinoma—mutations or fusions in the FGFR2 gene lead to uncontrolled cell proliferation.
Pemigatinib is designed to:
- Bind selectively to altered FGFRs
- Inhibit tumour growth driven by FGFR signalling
- Promote apoptosis (programmed cell death) in cancer cells
It’s approved for adult patients with previously treated, unresectable or metastatic cholangiocarcinoma with an FGFR2 fusion or rearrangement.
How Does Pemigatinib Work?
The FGFR signalling pathway regulates vital cellular functions. When FGFR2 mutations or fusions occur, they bypass normal cell regulation, resulting in constant signalling for cell growth—even in the absence of a stimulus.
Pemigatinib works by:
- Blocking the kinase activity of FGFR1-3
- Disrupting downstream signalling pathways that promote tumour survival
- Halting angiogenesis, the formation of new blood vessels that feed the tumour
This precision mechanism means the drug specifically targets cancer cells with FGFR2 alterations, sparing normal cells and reducing systemic toxicity.
PEMAZYRE: Pemigatinib’s Trusted Brand
In India, Pemigatinib is available as PEMAZYRE 4.5MG / 9MG / 13.5 MG TAB—a reliable and high-quality formulation designed to offer consistent dosing for oncology patients.
Each dosage strength (4.5 mg, 9 mg, and 13.5 mg) allows physicians to:
- Start therapy at the standard 13.5 mg once daily for 14 days in a 21-day cycle
- Adjust based on tolerance, side effects, or lab results
- Ensure patient-specific management with minimal interruptions
PEMAZYRE meets international manufacturing standards and is distributed through trusted medical supply channels across India.
Clinical Evidence: How Effective Is Pemigatinib?
Pemigatinib was granted accelerated approval based on the FIGHT-202 trial, a pivotal Phase 2 clinical study that evaluated its efficacy in patients with FGFR2-fusion positive cholangiocarcinoma.
Key Findings:
- Overall Response Rate (ORR): 35.5%
- Median Duration of Response (DoR): 7.5 months
- Disease Control Rate (DCR): Over 80%
- Median Progression-Free Survival (PFS): 6.9 months
- Median Overall Survival (OS): 21.1 months
These results were especially significant given the aggressive nature of the disease and the limited effectiveness of chemotherapy in this population. It marked Pemigatinib as a first-in-class FGFR inhibitor with real clinical value.
Approved Uses and Ongoing Research
Approved Indication:
- Adults with previously treated, unresectable, locally advanced or metastatic cholangiocarcinoma with FGFR2 gene fusions or rearrangements
Investigational Uses:
Pemigatinib is also being studied in:
- Bladder cancer (urothelial carcinoma) with FGFR3 alterations
- Solid tumours with FGFR gene mutations
- Combinations with immunotherapies and other targeted agents
If ongoing trials show similar benefits, Pemigatinib could soon be used in more cancer types with FGFR involvement.
Dosage and Administration
Pemigatinib is usually taken as:
- 13.5 mg orally once daily for 14 days, followed by
- 7 days off in a 21-day treatment cycle
Important notes:
- Taken with or without food
- Avoid strong CYP3A4 inhibitors/inducers
- Frequent monitoring of phosphate levels, liver function, and eye exams is essential
Dosage may be reduced or interrupted based on:
- Side effects like hyperphosphatemia or fatigue
- Abnormal lab results
- Tolerance over long-term use
Side Effects and Safety Considerations
Pemigatinib, while targeted, does have a side effect profile that requires active monitoring and patient education.
Common Side Effects:
- Hyperphosphatemia (high phosphate levels in the blood)
- Dry mouth and taste changes
- Nail and skin issues
- Hair loss
- Diarrhoea or constipation
- Fatigue and decreased appetite
Serious Side Effects:
- Retinal detachment or eye disorders (patients must have regular eye exams)
- Elevated liver enzymes
- Electrolyte imbalances
Most side effects are manageable with supportive care, phosphate binders, or dose modifications. Patient compliance and routine check-ups are key.
Patient Eligibility: Who Should Consider Pemigatinib?
Pemigatinib is only prescribed for patients with confirmed FGFR2 gene alterations. Therefore, molecular testing of the tumour tissue is required before initiating therapy.
Ideal candidates include:
- Adults with intrahepatic cholangiocarcinoma
- No surgical options or advanced disease
- Disease progression after at least one line of chemotherapy
- Confirmed FGFR2 fusions or rearrangements
Patients must be carefully screened and monitored to ensure treatment safety and efficacy.
The Importance of Genetic Testing
FGFR alterations are not visible through routine pathology. Instead, next-generation sequencing (NGS) or FISH (fluorescence in situ hybridization) is used to detect these specific mutations.
Genetic testing helps:
- Identify candidates for Pemigatinib therapy
- Avoid unnecessary treatments
- Improve clinical outcomes through targeted intervention
This highlights the growing importance of precision medicine and the integration of molecular diagnostics into oncology care.
Cost and Accessibility in India
While Pemigatinib is a high-value targeted therapy, cost and access are important considerations. The availability of PEMAZYRE 4.5MG / 9MG / 13.5 MG TAB ensures that Indian patients can receive authentic, clinically approved medication without the need for international import.
Key benefits include:
- Availability in major cancer centres and pharmacies
- Support from oncologists experienced in targeted therapy
- Dosing flexibility for long-term tolerability
Financial assistance programs and generic alternatives may be available to further reduce the treatment burden.
The Future of FGFR-Targeted Therapies
Pemigatinib is just the beginning. The FGFR pathway is increasingly recognised as a major driver in several difficult-to-treat cancers. Drugs like Pemigatinib show that it’s possible to halt cancer by cutting off its growth signals at the source.
As research continues, expect to see:
- More indications added for FGFR inhibitors
- Combination regimens with immunotherapy
- Development of second-generation inhibitors with improved tolerability
The era of broad, toxic chemotherapy is giving way to precise, mutation-specific therapies like Pemigatinib that offer new hope without compromising quality of life.
Final Thoughts
Pemigatinib is a breakthrough targeted therapy offering real progress in the treatment of FGFR2-altered cholangiocarcinoma. Its effectiveness, manageable safety profile, and oral convenience make it a critical option for patients with previously limited hope.
Indian patients can now access Pemigatinib easily through reliable formulations like PEMAZYRE 4.5MG / 9MG / 13.5 MG TAB, bringing cutting-edge global oncology solutions closer to home.
If you or a loved one has been diagnosed with advanced cholangiocarcinoma, consult your oncologist about FGFR testing and whether Pemigatinib could be the next step forward.